What a great year we had at Thryv!
During 2022, we expanded the team and are now at 16 full time employees.
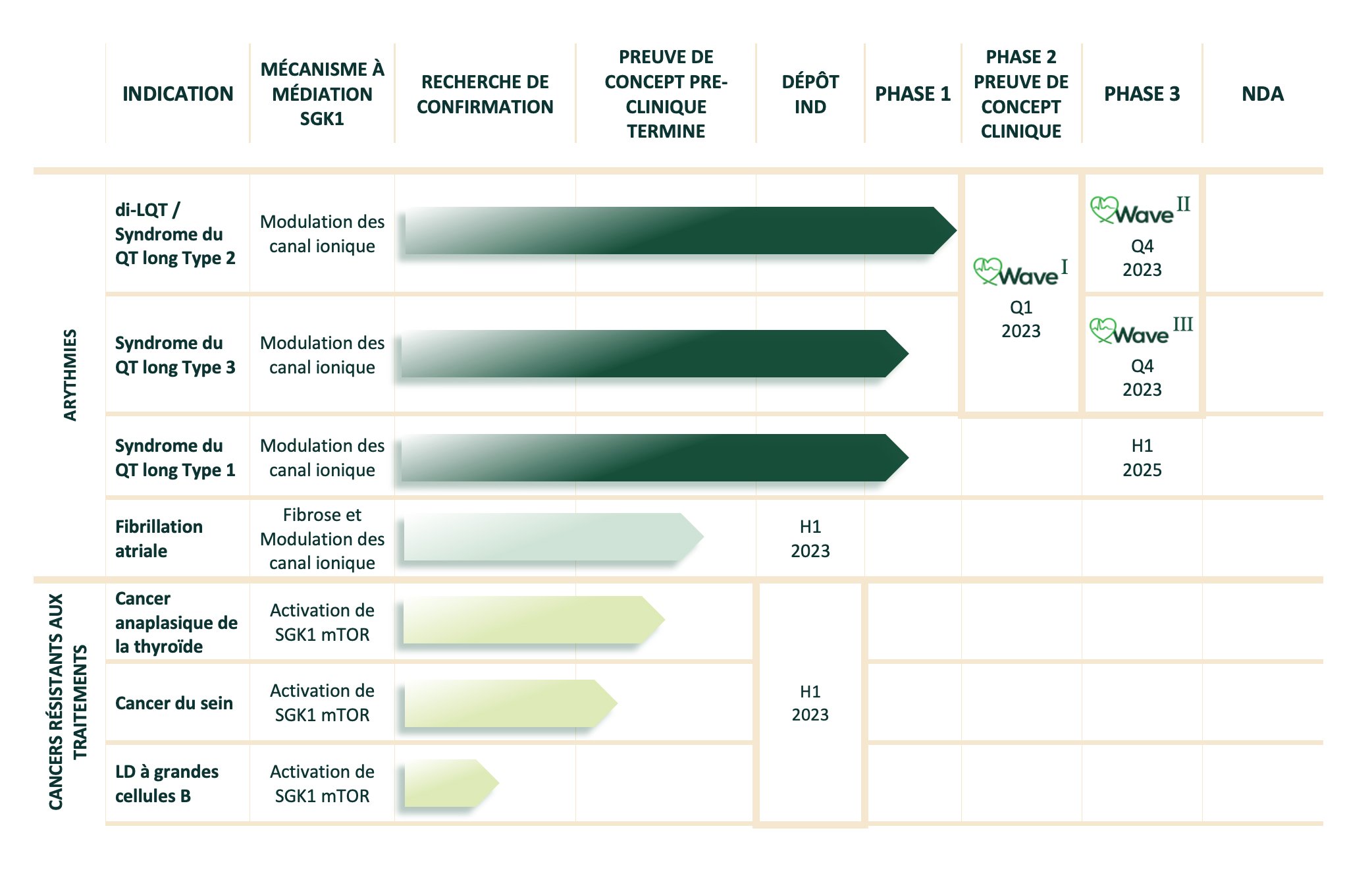
We advanced our lead candidate for Long QT Syndrome to IND and completed the first part of our Phase 1 trial.
We entered into collaborations with major academic centers for our oncology program in treatment-resistant cancers.
On a sad note, our inspiration to break barriers and move as fast as we can to advance our Long QT Syndrome program, lost her battle with Long QT Syndrome in late 2022. Isla Hutton passed away just prior to her 7th birthday due to complications from her disease.
Our commitment to find a therapy for children like Isla has never been stronger and we are humbled by the struggles of patients and families who live with this terrible disease.
2023 is shaping up to be a phenomenal year and we expect to have multiple data readouts that will hopefully be transformative, including:
Our final readout from our Phase 1 safety study
Data from our Long QT Syndrome Phase 2 study
Initiation of our Patient Engagement Program "Catch the QT Wave" where we hope to change the lives of patients with Long QT Syndrome Type 2 and Type 3
Exploratory data from mice in an obese model of atrial fibrillation
Exploratory data from various on-going in vivo models of advanced breast cancer and anaplastic thyroid cancer.
Wishing all our supporters, patients and families a healthy and happy new year!
Debra Odink
President & Chief Development Officer